1.Purpose of Investigation
The "Guidelines on the Use of Radiocommunications Equipment such as Cellular Telephones and Safeguards for Electronic Medical Equipment" was developed at Electromagnetic Compatibility Conference Japan in March, 1997. Since then, the Ministry of Internal Affairs and Communications has continued to carry out investigations to reduce risks to the public associated with radio wave use and to secure the radio-wave environment for wide and safe use of mobile telephone terminals and other equipment. Results of the studiees are made public.
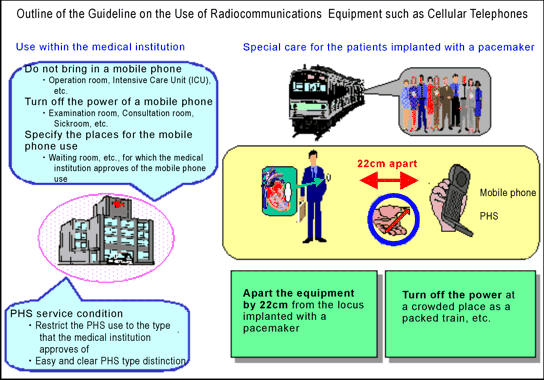
The following illustration shows the outline of the "Guidelines on the Use of Radiocommunications Equipment such as Cellular Telephones and Safeguards for Electronic Medical Equipment".
2.Method of Investigation
- i.Target Equipment
-
Medical Equipment
The implanted pacemaker and the implanted defibrillator (medical equipment implanted into a patient's body for the purpose of treatment in a similar way to the pacemaker) were two categories of targeted medical equipment for the investigation. Here, the aim is consistency with the results of the investigation concerning the influence on the implanted pacemaker conducted at the Electromagnetic Compatibility Conference Japan between 1995 and 1996, and to make an accurate comparison of the results of both investigations. -
Wireless Equipment
As for the targeted mobile telephone types, PHS, cdmaOne, and IMT-2000 were selected among the PDC-mode mobile telephones (800MHz band, 1.5GHz band).
-
- ii.Investigation Overview
The investigation was based on the method described in the "Report of Investigation on the Use of Radiocommunications Equipment such as Cellular Telephones - Safeguards for Electronic Medical Equipment" published by the Electromagnetic Compatibility Conference Japan in April, 1997.
The investigation was conducted entirely in a radio anechoic chamber, keeping the pacemaker and the defibrillator under the same condition as when implanted in the human body, subjecting them to radio wave exposure from a mobile telephone, then observing the influence.
In the observation, the distance between the mobile telephone and the pacemaker and/or the defibrillator was changed to record influences at various distances.
Because the output of a mobile telephone is controlled by commands from the base station, it does not always emit a radio wave of optimum electrical power. Also, the electrical power was not consistently stable in every investigation cycle, because the radio wave from the base station could not reach the radio anechoic chamber investigation site. For this reason, a special controller was installed to emit a stable optimum electrical power all the time and to maintain investigation data repeatability.
3.Result of Investigation
As a result of this investigation, the current guideline ("A person with an implanted pacemaker should use or carry a mobile telephone terminal at a distances more than 22cm from the site of implantation," as announced by the Electromagnetic Compatibility Conference Japan in March, 1997) was again proved to be appropriate, and the same guideline was applied to an implanted defibrillator.
- No implanted pacemaker was influenced exceeding the maximum interference distance of 15cm in the previous investigation (conducted between FY 1995 and FY 1996), showing apparentimprovement on the whole.
As evidence of this, the result of the investigation in the 800MHz band and 1.5GHz band PDC mode mobile telephone terminals can be referenced as an attachment. - The distance of 22cm, the safe distance described in the current guideline (the safety factor is considered against the maximum interference distance of 15cm), was not to be altered, and it was regarded unnecessary to alter it in any case.
