1.Outline
Recently, a variety of wireless systems including the mobile phone are playing a more important role in our daily lives, and the opportunity to use radio equipment is also increasing.
The use of radio equipment includes PHS (Personal Handyphone System) handset and wireless card (contactless IC card) system, electronic articles surveillance equipment, RFID (electronic tag) equipment, and wireless LAN equipment, etc. in addition to the mobile phone handset.
When the use of radio equipment and electrical and electronic equipment are in close proximity, the potential of malfunction may occur in electrical and electronic equipment due to the radio emissions from the use of radio equipment.
When such a malfunction occurs by the radio emissions from the use of radio equipment, there is a possibility that an implanted medical device could produce an adverse effect on human health.
It is therefore important to share essential information about the incidence of such effects and prevention between the users of radio equipment and those who have a medical device implanted in them.
As information on the incidence and prevention of the effect on implanted medical devices, the "Guidelines on the Use of Radiocommunications Equipment such as Cellular Telephones and Safeguards for Electronic Medical Equipment" was enacted in 1997 at the Electromagnetic Compatibility Conference Japan. (Consist of experts in academia, related ministries and agencies in the government, and related industry organizations.)
The Ministry of Internal Affairs and Communications has continued to carry out investigations to reduce risks to the public associated with radio wave use and to secure the radio wave environment for wide and safe use of mobile phones and other equipment, and has published the Guidelines to safeguard people with implanted medical devices from adverse effects.
The following outlines the "Guidelines on the Use of Radiocommunications Equipment for Implanted Medical Devices" (enacted in August 2005, are revised in April 2007).
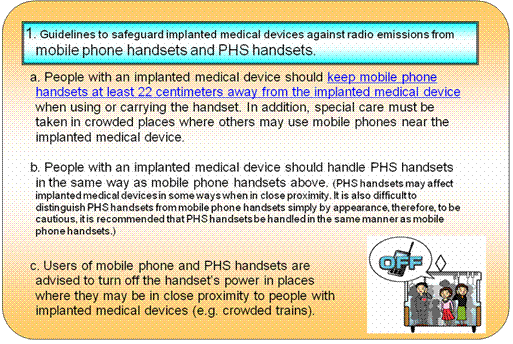
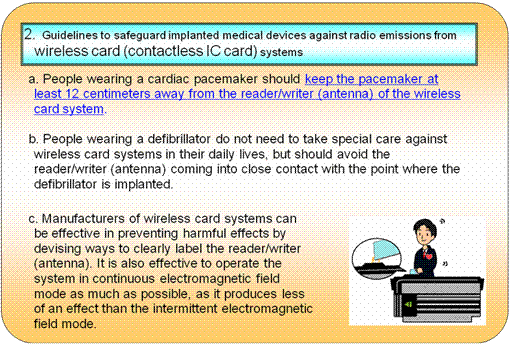
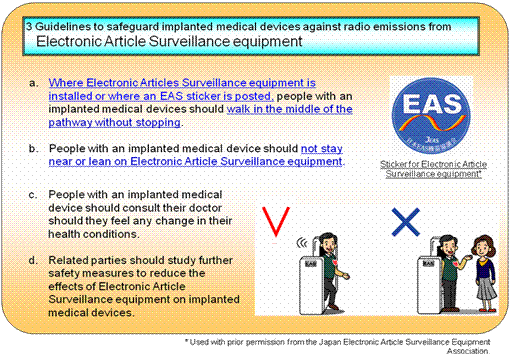



2.Result of Investigation
3.Reference
Investigation into the Use of Radilcommunication Equipment such as Mobile Phones and Safeguards for Electronic Medical Equipment(Electromagnetic Compatibility Conference, 1995-1996)
